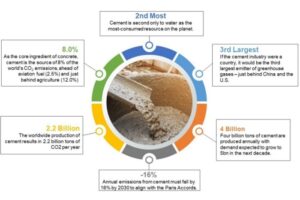
The manufacturing process which produces ordinary portland cement, the ubiquitous ingredient in the concrete used around the world, is a major contributor to greenhouse gas emissions. Portland cement is the gray powder binder that mixes with water to form concrete, and manufacturers make about 5 billions tons of the stuff each year. For every ton of cement produced, the process creates approximately a ton of carbon dioxide, all of which accounts for roughly 7 percent of the world’s carbon dioxide emissions.
Economic studies indicate that the demand for concrete and thus portland cement will continue to grow each year, which means that anything that can be done to lessen the environmental impact of this production is a welcome step, and could even be a substantial money saver for companies that are required to pay carbon-emissions taxes.
Gaurav Sant is an associate professor of civil and environmental engineering at University of California – Los Angeles and UCLA’s Edward K. and Linda L. Rice professor of materials science. He’s also a scientist with California NanoSystems Institute at UCLA who recently completed research that could eventually lead to methods of cement production that give off no carbon dioxide, the gas that composes 82 percent of greenhouse gases.
Sant has found that carbon dioxide released during cement manufacture could be captured and reused. The new research has been published in the journal Industrial and Engineering Chemistry Research.
Two steps in cement manufacturing are responsible for carbon emissions. To create cement, the raw material limestone is heated to about 750 degrees Celsius. This process, known as calcination, separates limestone into calcium oxide, or what’s known as lime, and carbon dioxide gas. Lime is a corrosive and unstable solid, but when combined with water, a process called slaking, it forms a more stable compound called calcium hydroxide. Sixty-five percent of the carbon dioxide emitted during the production of cement comes from this calcination process.
A material known as tricalcium silicate is the major compound in portland cement. This material hardens like stone when it is combined with water, which is what makes cemet the perfect binder. Tricalcium silicate is produced by combining lime with silica-containing sand and heating the mixture to 1,500 degrees Celsius. Thirty-five percent of the carbon dioxide emitted during the production of cement is given off by this process of heating the tricalcium silicate compound.
Sant and his team showed that the carbon dioxide given off during calcination can be captured and recombined with calcium hydroxide to recreate limestone — creating a cycle in which no carbon dioxide is released into the air. In addition, about 50 percent less heat is needed throughout the production cycle, since no additional heat is required to ensure the formation of tricalcium silicate.
The laboratory research that Sant and his colleagues have done was conducted on a small sample size, but Sant believes that the time is right to move into the mainstream with this idea.